Reactions
Scientifically the most common and studied reactions are the Chemical Reactions. It is a process that results in interconversion of different substances. The substances involved in these kind of reactions are called Reactants. These reactants react under a given circumstance and undergo a chemical change. The results often produce one or more compounds, which are absolutely of different nature to those of the reactants.
Let us take a very common example. We take two substances: wood and fresh air. Wood is fuel and fresh air is Oxygen. We make the environment around the wood very hot by lighting up a fire. The wood starts burning. After it is completely burnt, we get a grayish black ash and choking grayish fumes. Now, wood is solid brown and fresh air is transparent and easy to breathe. But after the reaction, at the end, we get grayish black ash which is different from wood and the choking fumes which is totally opposite to the fresh air we breathe. Therefore, wood + oxygen = fly ash + carbon-dioxide + unburnt particles.
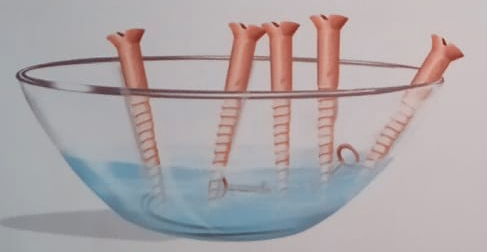
Another examples is iron screws reacting with water and air. The solid shining iron screws wen react with moist air turn into rusty brittle substance.




Comments
Post a Comment